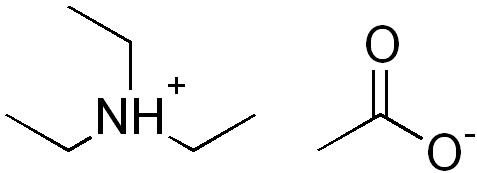
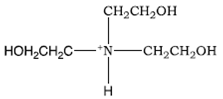
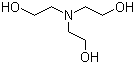
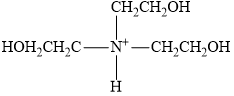
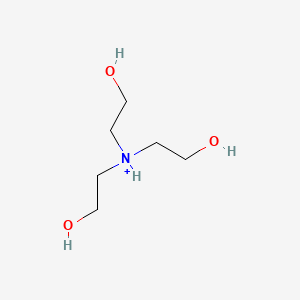
SOLVED: How to Prepare A Triethanolamine Buffer Shown hete is the structure Ol (riethanolamine in Its tully protonated orm; GH,CHLOH HOCH CH, CHCHOH Its pK, is 7. , You have available at
Triethanolamine hydrochloride | Biological Buffer Reagents | Biochemistry | Life Science | Carl Roth - International
![PDF] Formulation and some biological uses of a buffer mixture whose buffering capacity is relatively independent of pH in the range pH 4-9. | Semantic Scholar PDF] Formulation and some biological uses of a buffer mixture whose buffering capacity is relatively independent of pH in the range pH 4-9. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/dd0a648b5ba29c90ffad9154d9db8c3b5af10cfc/2-Table1-1.png)
PDF] Formulation and some biological uses of a buffer mixture whose buffering capacity is relatively independent of pH in the range pH 4-9. | Semantic Scholar

Triethanolamine, (HOCH2CH2)3N, is a widely used biological buffer, with maximum buffering capacity at pH 7.8. Propose a synthesis of this compound from ethylene oxide and ammonia. | Homework.Study.com

5. CV of 0.02M l-dopa in Triethanolamine buffer (pH 10), scan rate 50 mV/s | Download Scientific Diagram